External quality control programs for Viral Hepatitis detection methods
Dear Partners and Friends,
Hepatitis is known as one of the most common infectious diseases in the world. It is characterized by great social significance due to the high risk of developing cirrhosis of the liver or liver cancer.
It has been found that in many countries, more than 80% of the population has contracted Hepatitis A. According to the World Health Organization, about 2.5 billion people have contracted Hepatitis B, with 500 million remaining permanently infected. About 200 million people are infected with Hepatitis C.
Viral Hepatitis is often asymptomatic and timely and correct diagnosis can prevent severe liver damage and save millions of lives.
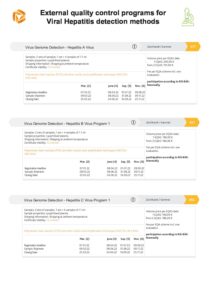
In the attached file you will find more information about the following programs for external quality control for Viral Hepatitis detection methods of INSTAND:
- Virus Genome Detection – Hepatitis A Virus – 377
- Virus Genome Detection – Hepatitis B Virus Program 1 – 361
- Virus Genome Detection – Hepatitis C Virus Program 1 – 362
- Virus Genome Detection – Hepatitis E Virus – 380
- Virus Genome Detection – Hepatitis A Virus (Ab) – 343
- Virus Genome Detection – Hepatitis C Virus (Ab and HCV Antigen) – 346
- Virus Genome Detection – Hepatitis D Virus (Ab) – 347
- Virus Genome Detection – Hepatitis E Virus (Ab) – 348
- Virus Immunollogy – Hepatitis B Virus Program 1 (HBsAg, Anti-HBs, Anti-HBc) – 344
- Virus Immunology – Hepatitis B Virus Program 2 (Anti-HBc-IgM, HBeAg, Anti-HBe) – 345
The final date for registration for all programs in the next cycle is 08.04.2022!
The indicated prices are in EURO and do not include VAT.
Transport costs are paid separately and distributed among the participants.
Do not hesitate to contact us with additional questions or if you would like to participate in the external control programs of INSTAND!
Best regards,
Seda Ahmedova
